Fill your tires with Nitrogen. Nitrogen helps tires maintain the right pressure for a longer period of time as opposed to oxygen, this because nitrogen molecules are larger than oxygen molecules. So the next time you need to add air to your tires, add nitrogen.Immediately I was skeptical. Oxygen is the 8th element on the periodic table, whereas nitrogen is the 7th; oxygen has a higher atomic mass than nitrogen. So can an O2 molecule really be smaller than an N2 molecule?
I had Google help me out and I found this paper. If you ask me, this guy is trying too hard to sound smart, like when people say "I" instead of "me." I mean come on, he didn't bother to subscript his 2s when writing O2! ...Not to mention the paper is hosted on getnitrogen.org, which is the website for the Get Nitrogen Institute. (Institute?! Really??) Their whole idea is to get you to put nitrogen in your tires. I'm sure they're in some way financed by nitrogen people.
I found this on Answers.com (just as dubious as the nitrogen people):
Molecular size a bit tricky. As a quick comparison, we can use the covalent radius defined as 1/2 the distance between to identical covalently bonding nuclei. This is measured in picometers (1 pm= 1x 10-12 m). Nitrogen's covalent radius is 75pm so the length of a nitrogen (N2) molecule ought to be 4 X 75pm or 300 pm. A molecule of oxygen (O2) ought to be just a shade smaller 4 X 73pm or 292pm. So an oxygen molecule is a little less than 3% smaller than a nitrogen molecule.
So, I did some more searching and came across this.
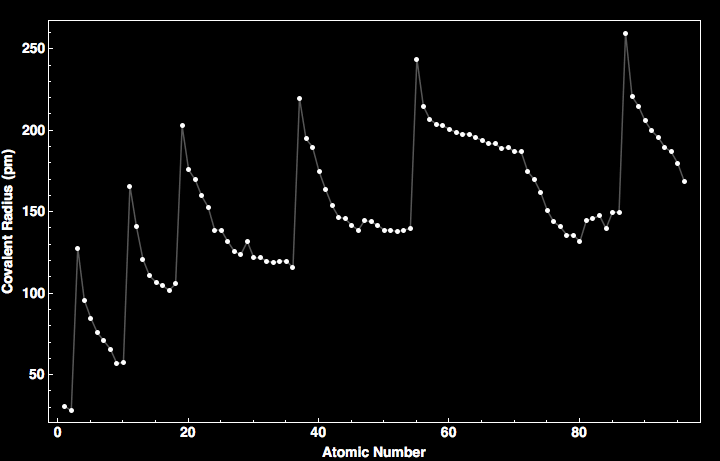
As you can see, covalent radii generally decrease across periods in the Periodic Table. And now all that high school chemistry is coming back to me.
Oh, yeah! Chemistry sucks!
So anyway, with some assumptions, I can agree that yes, N2 molecules are smaller than O2 molecules.
But let's back up! The whole point of this was what to fill your tires with! Who is filling their tires with oxygen?! If a tire caught fire in a wreck IT WOULD EXPLODE if it were filled with oxygen! They're filled with AIR, which is 78% nitrogen! I find it extremely hard to believe that using pure nitrogen to get that last 22% of non-nitrogen out of the air in the tires is going to be worth the time, effort, and money. Just check your tire pressure regularly, damnit.
Most of the other tips check out, but some of that original article is crap. For example, "Shopping around for cheaper gas DOES NOT burn more than you’ll end up saving." That depends on how far you need to drive to shop around, doesn't it? "Gas is NOT cheaper mid-week." Depends on the gas station, doesn't it? "Opening the windows instead of using the air conditioner has no measurable effect." No measureable effect?? Then what did the MythBusters measure?! (I'll save you the Google search: It is more fuel efficient to use air conditioning when the car is traveling approximately 50mph or more. Otherwise, windows are more fuel efficient.)


No comments:
Post a Comment